Nature Communications 8352 (2015), DOI:10.1038/ncomms9352
A Recursive Vesicle-based Model Protocell with a Primitive Model Cell Cycle
Kensuke Kurihara, Yusaku Okura, Muneyuki Matsuo, Taro Toyota, Kentaro Suzuki, Tadashi Sugawara*
News
Home
TOPIC

Nature Communications 8352 (2015), DOI:10.1038/ncomms9352
A Recursive Vesicle-based Model Protocell with a Primitive Model Cell Cycle
Kensuke Kurihara, Yusaku Okura, Muneyuki Matsuo, Taro Toyota, Kentaro Suzuki, Tadashi Sugawara*
| Tadashi SUGAWARA | Dept. of Chem., Facl. of Sci., Kanagawa Univ. | |
| Room 2-217 sugawara-t[at]kanagawa-u.ac.jp | ||
| http://www.chem.kanagawa-u.ac.jp/~sugawara/ |
 "How a life emerged on the earth?", "What is an intrinsic nature of a life?", "What is the difference between a life and a material?" are the questions raised by everybody. Because a life is made of a cell, we can explore the mystery of life if we could prepare a model protocell chemically. Such researches as synthesizing a life from materials have recently been attracted much attention over the world.
"How a life emerged on the earth?", "What is an intrinsic nature of a life?", "What is the difference between a life and a material?" are the questions raised by everybody. Because a life is made of a cell, we can explore the mystery of life if we could prepare a model protocell chemically. Such researches as synthesizing a life from materials have recently been attracted much attention over the world.
We succeeded in the construction of a giant vesicle (GV)-based model protocell which self-proliferates over generations just like a living cell. His group has already reported a liked proliferation between a replication of DNA as a model of the gene and a self-reproduction of GV as a model of cell membrane (Nature Chem. 2011, Movie). However the divided GV of the 2nd generation cannot replicate DNA any longer because of the depletion of substrates (deoxynucleotides) in the GV.
In the current study, his group found a method to replenish depleted substrates or enzymes into an empty GV through adhesion and fusion between an empty daughter GV and a conveyer GV filled with substrates, triggered by a pH-jump. The resulted proliferation loop consisted of four discrete phases (Maturation, Division, ingestion, replication). The proliferation over generations guarantees an appearance of a mutant which may become a predominant species for evolution.
These results give a clue to unveil the mystery how a life was born from materials in the prebiotic earth and how a life acquired a primitive mechanism of evolution.
Constructive Approach to a Model Protocell
This figure illustrates a road map to construct a model protocell chemically using well-defined organic molecules and macromolecules. First, simplify the structure of a cell as simple as possible, leaving the intrinsic properties untouched. Second, construct a protocell using three indispensable elements, a compartment (vesicle) which separates the inner reaction system from the outer world, a catalyst (enzyme) which catalyzes important metabolic reactions and an informational substance (RNA/DNA), which delivers the characteristic of an original protocell to its descendant. Third, establish a linkage between a self-production of a vesicle, and a self-replication of DNA, to lead a linked proliferation of a model protocell.
Hierarchical Dynamics of a Model Protocell
This figure describes two complimentary concepts, a biomolecule-based view (above) and a collaborative dynamics view (below) to explain the origin of life. In the biomolecule-based view, the origin of life is ascribed to a RNA world or a protein world. In the collaborative dynamics view, an essence of a life is characterized not by sophisticated biomolecules but by hierarchical dynamics derived from the interaction between elemental kinetic processes and morphological changes of a GV-based protocell, such as linked proliferation between reproduction of a compartment and replication of an information substance, and a recursive proliferation with a cell cycle, and evolution arisen from the appearance of a mutant after repeated proliferations.
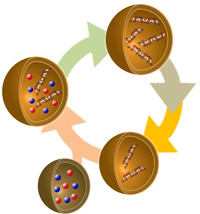
Primitive Model Cell Cycle of Recursive Proliferation of a Model Protocell
The recursive proliferation is characterized by a primitive cell cycle consisting of four discrete phases (maturation, division, ingestion, and replication). A linked proliferation between a reproduction of giant vesicle (GV) and a replication of DNA in GV was reported in the previous work. Newly born vesicles, however, could not replicate DNA because the substrates for DNA amplification was depleted. The proliferation loop was completed by inserting an Ingestion phase between a Division phase and a Replication phase by introduction of a GV delivery system using a conveyer GVs filled with depleted substrates.
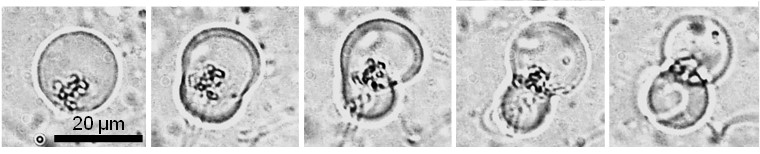
Discrete Four Phases in a Primitive Model Cell Cyclel
Four discrete four phases in a primitive model cell cycle are shown in this figure. In an ingestion phase, two types of GVs, a daughter GV with depleted substrates and a conveyer GV filled with substrate (deoxynucleotides), adhered and fused because the surface charges of these two types GVs become opposite by lowering pH (pH-jump) of the dispersion. Then, the depleted substrates are transferred into the empty daughter GV, and the amplification of DNA restarted in a replication phase under thermal cycles. Amplified DNA intrudes into the vesicular membrane by being coated by cationic membrane lipids and it forms a complex with amphiphilic catalysts, forming an active site, in a maturation phase. Production of membrane lipids from its precursor occurs predominantly around the active site, which induces a budding deformation and division in a division phase.
Scope of Repeated Proliferation of a Model Protocell
Since the DNA sieves as a pseudo-enzyme in this protocell, it influences the mode of GV division, which makes a biological distance between geno-type and phenotype closer. Then, the recursive proliferation over generations leads to an appearance of mutants which may become a predominant species through a natural selection.
| References |
|---|
| Related Materials |
|---|
Sept30,2015 Suzuki K.